Critical care research
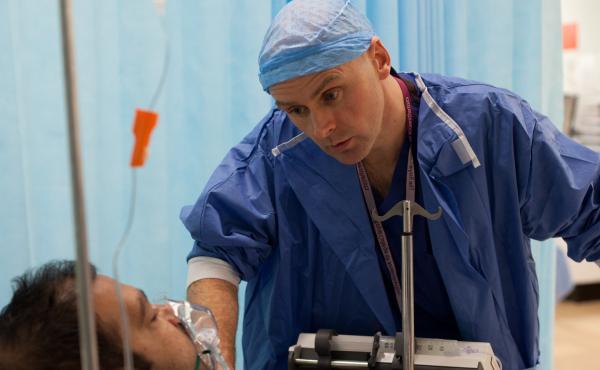
Some Critical Care Units actively participate in patient research
Conducting strictly regulated and carefully monitored research trials offers the opportunity to improve the way patients are treated and cared for.
Research trials enable us to safely evaluate new treatments or equipment and answer whether they may benefit future patients. Answers from research trials will continue to improve important outcomes for patients, such as survival free from disability.
Regulation
Research projects are very carefully regulated and the protection of all patients involved is the primary concern. Before any study can be started independent ethics committees have to approve the study. Each study proposal is reveiwed in great detail by experts to ensure that that the study planned is not only safe but is genuinely testing something we do not already know.
Participating in research
A Research Nurse reviews whether patients are eligible to be enrolled into a critical care trial. If this is the case, having discussed it with the patient’s consultant, they may approach the patient or if the patient cannot communicate, their next of kin. They will be given all relevant information about the research trial and time to consider whether they want to take part or not. Participation in medical research projects is voluntary. If you or your family decide that you do not want to take part this will not affect your other care in any way.